Introduction
Refractory or relapsed (R/R) multiple myeloma (MM) is often associated with a poor prognosis following chemotherapy or even transplantation. BCMA-targeted CAR-T therapy has demonstrated notable efficacy in R/R MM. In the past, the overall response rate (ORR) was relatively low at around 60%, but in recent years, advancements in the manufacturing of BCMA-CAR-T products have led to a substantial improvement in efficacy. Here we explore the efficacy and safety of nanobody-based anti-BCMA CAR-T cell therapy in R/R MM from a clinical trial (https://clinicaltrials.gov NCT04447573).
Methods
Autologous peripheral blood lymphocytes were collected from patients, who subsequently underwent intravenous fludarabine (30mg/m 2/d) and cyclophosphamide (300mg/m 2/d) lymphodepletion chemotherapy from day-5 to day -3. The SL-BCMA CAR molecule, an anti-BCMA CAR-T cell product generated by Senlang Biotechnology, features a structure that includes dual nanobody VHHs targeting BCMA (dVHHs), CD8α hinge region, CD8α transmembrane domain (TMD), co-stimulatory domain (4-1BB), and CD3 zeta domain (CD3ζ). Among them, dVHHs consist of two different VHHs connected by short peptides. The CD8α hinge region provides an effective spatial architecture for the binding of dVHHs and BCMA protein. The first signal of CAR-T cell activation is completed along with the intracellular CD3ζ domain. The 4-1BB intracellular co-stimulatory domain provides co-stimulatory signals for the activation of CAR-T cells. Under the dual signal stimulation, CAR-T cells are activated and expanded. After the BCMA CAR-T cells infusion on day 0, patients underwent evaluations, including immunoglobulin fixed electrophoresis, free light chains in blood and urine, bone marrow penetration, and whole-body PET CT to evaluate the efficacy at the first month, second and third month, and every three months thereafter.
Results
From April 2021 to May 2023, 21 R/R MM patients in our hospital were treated with SL-BCMA CAR-T, including 10 males and 11 females. The median age is 57 years (30-73 years). Among them, there were 2 cases with Plasma cell leukemia, 10 had extramedullary multiple lesions, and 2 had paralysis caused by central nervous system infiltration. Pre-CAR-T, 11 patients had a prior transplant history including 10 who relapsed after autologous transplantation and 1 who relapsed after allogeneic transplantation. One patient had a rare type of anaplastic Plasma cell tumor. Seven patients presented high-risk factors characterized by FISH abnormalities such as t (4; 14) (p16; q32), t (14; 16) (q32; q23), and 3 had TP53 mutations. The median BCMA expression rate among 19 patients was 53.3% (range: 18.5% -100%), while the remaining 2 patients displayed non-BCMA expression. The median BCMA CAR-T cell dose was 1*10 6/Kg (0.3-2*10 6/Kg).
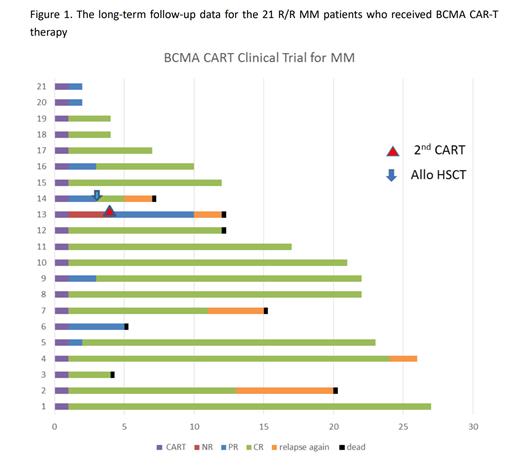
One month following BCMA-CAR-T cell infusion, the ORR was 95%(20/21), and the CR rate was 38% (8/21). The patient who initially showed no response achieved a partial response following a second infusion of BCMA-CAR-T cells. One died at about 2.5 months due to lung infection. Evaluations conducted at the three-month mark showed an ORR of 95% (19/20) and a CR rate of 80% (16/20). One patient relapsed at the second month and underwent salvage allogeneic hematopoietic stem cell transplantation. For the long follow-up data, the median duration of remission was 11 months. Of the 7 deaths, 2 were attributed to infection during disease control, and 5 were due to disease progression. (Figure 1)
In terms of side effects, 6 patients developed grade 2 cytokine release syndrome (CRS), and only 1 patient developed grade 3 CRS. Only one patient had grade 1 neurotoxicity.
Conclusions
The clinical trial demonstrated that BCMA CAR-T therapy, composed of dual nanobody VHHs targeting BCMA (dVHHs), exhibits a high ORR with a manageable safety profile in treating R/R MM patients. This extends even to high-risk patients, such as those with extramedullary lesions, cytogenetics high-risk groups, and patients with plasma cell leukemia or anaplastic plasma cell tumor.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal